What is Patient Information Booklet?
The Patient Information Booklet provides data from FDA clinical trials including risks, contraindications, warnings and results. The FDA requires that every patient who is about to undergo LASIK surgery be given the Patient Information Booklet published by the laser manufacturer. Non-compliance with this FDA mandate is widespread. Each FDA-approved laser has an approval order with the Patient Information Booklet mandate. Lasik Doctors hide booklets from patients and 99,99% of patients don't receive those before lasik. In this blog you will see a compilation with tables from Patient Information Booklets. They all are available on the fda web page. This is the most official information about lasik. If patients were receiving Patient Information Booklets before surgery, most wouldn't do lasik. Patient Information Booklets show a high number of 'symptoms' (Dry eyes, halos, starbusts, light sensitivity, glare, double vision.....), that's the main reason why lasik doctors hide booklets from patients. LASIK manufacturers and their collaborators successfully pressured FDA to classify these problems as mere “symptoms” so that manufacturers could claim that the adverse event rate is less than one percent.
Below are three examples of the FDA mandate regarding Patient Information Booklets. The first example is found in an FDA Approval Order for the VISX laser, the second is found in the VISX Physician Instruction Booklet and third is found in an FDA premarket approval laser.
"Prospective patients, as soon as they express an interest in wavefront-guided LASIK for myopic astigmatism and prior to undergoing surgery, must receive from the treatment provider the Patient Information Booklet (as described in your final submission to this PMA supplement)." Link
"All patients must be given the opportunity to read and understand the Patient Information Booklet and to have all their questions answered to their satisfaction before giving consent for Laser Assisted In Keratomileusis (LASIK). " Link
"In advance of surgery, all prospective patients must receive the Patient Information Booklet (as described in your final submission to this PMA) from their treatment providers." Link
Example of an FDA Patient Information Booklet Cover from the laser Alcon Ladar6000.
LASIK manufacturers and their collaborators successfully pressured FDA to classify these problems as mere “symptoms” so that manufacturers could claim that the adverse event rate is less than one percent
Morris Waxler, PhD. From 1996 to 2000 was the FDA's chief scientist in charge of the clinical trials research for laser eye surgery.
http://www.lasikcomplications.com/Waxler_petition_FDA_stop_LASIK(6Jan11).pdf
http://www.lasikcomplications.com/Waxler_petition_FDA_stop_LASIK(6Jan11).pdf
"FDA originally counted glare, halos, dry eye, night driving difficulties, and similar problems after excimer laser refractive surgery as adverse events, e.g. page 16 of the Patient Information Brochure for P970053c says “…adverse events beyond the first few months: night vision difficulty (48.1% at six months)…glare (34.4% at 6 months)…” LASIK manufacturers and their collaborators successfully pressured FDA to classify these problems as mere “symptoms” so that manufacturers could claim that the adverse event rate is less than one percent."
"LASIK manufacturers and their collaborators emphasized “patient satisfaction” to divert FDA attention from continuing LASIK-patient complaints about glare, halos, dry eye and night driving problems."
"To this moment they and their collaborators have been successfully engaged in a pattern of falsifying, misrepresenting, manipulating, and withholding safety and effectiveness data from FDA to make their LASIK devices appear safer than they are."
Patient Information Booklets - 20/20 Vision with Worse 'Symptoms'
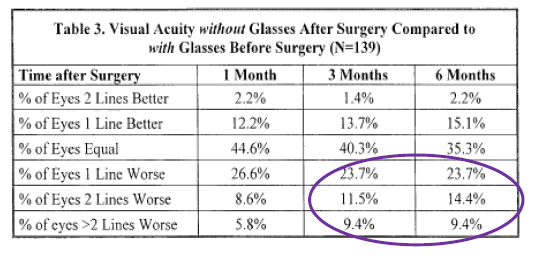
LASIK surgery is promoted based on the eye chart. Like the general public, prospective LASIK patients believe that 20/20 means good vision. LASIK surgeons are happy to discuss LASIK outcomes in terms of how well a patient can read the letters on the eye chart without glasses. But LASIK surgeons don't want to talk about how 20/20 vision may be bad after LASIK. FDA-required clinical trials demonstrate that LASIK have a high number of complications, even with the latest FDA-approved technology.
20/20 Vision with Worse night vision, Worse Starbusts, Worse Halos
20/20 Vision with Worse Double Vision, Worse Ghost
20/20 Vision with Worse Dry Eyes
There are no images to show ''Worse Dry Eyes', but you can simulate it by stopping to blink. After about 20 seconds you will start feeling Dry Eyes. This is 'worse dry eyes' as the lasik industry calls it.
Patient Information Booklets Compilation
This is how you can get Patient Information Booklets from FDA web page.
http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/SurgeryandLifeSupport/LASIK/ucm168641.htm
http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/SurgeryandLifeSupport/LASIK/ucm168641.htm
Download Patient Information Booklet Compilation
Subscribe to:
Comments (Atom)